Automated external defibrillator
From OHO - search engine for sustainable open hardware projects
|
Automated external defibrillator Basic Data Category: Health URL (first publication): https://github.com/CentroEPiaggio/Open-Automated-External-Defibrillator
Project status:
Technical documentation Maturity of the project:
no yes
Project management Versioning System:https://github.com/CentroEPiaggio/Open-Automated-External-Defibrillator Open-o-meter: 1 Product category: Business & Industrial
|
|
Description
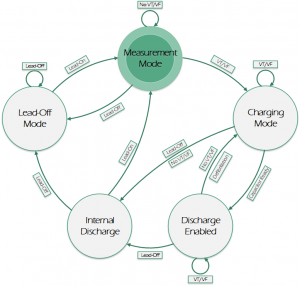
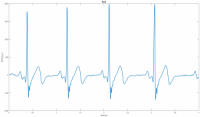
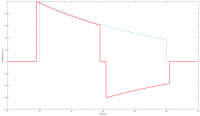
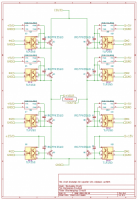
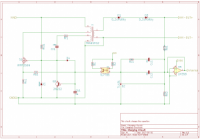
Automated External Defibrillator (AED) is a medical device that analyzes a patient's Electrocardiogram (ECG) in order to establish whether he/she is suffering from the fatal condition of Sudden Cardiac Arrest (SCA), and subsequently allows the release of a therapeutic dose of electrical energy (i.e. defibrillation). SCA is responsible for over 300'000 deaths per year both in Europe and in USA, and immediate clinical assistance through defibrillation is fundamental for recovery. In this context, an open-source approach can easily lead to improve the distribution and efficiency of AEDs. The proposed Open Source AED (OAED) is composed of two separate electric boards: a high voltage board (HV-B), which contains the circuitry required to perform defibrillation and a control board (C-B), which verifies SCA in the patient and controls the HV-B. Computer simulations and preliminary tests show that the OAED can release a 200 J biphasic defibrillation in about 12 seconds and recognizes SCA with sensitivity higher than 90% and specificity of about 99%. The OAED was also conceived as a template and teaching tool in the framework of UBORA, a platform for design and sharing medical devices compliant to international standards.
+ General Reviews